 Chapter 2 Self Quiz: The chemistry of biology
Chapter 2 Self Quiz: The chemistry of biology 
 Chapter 2 Self Quiz: The chemistry of biology
Chapter 2 Self Quiz: The chemistry of biology 
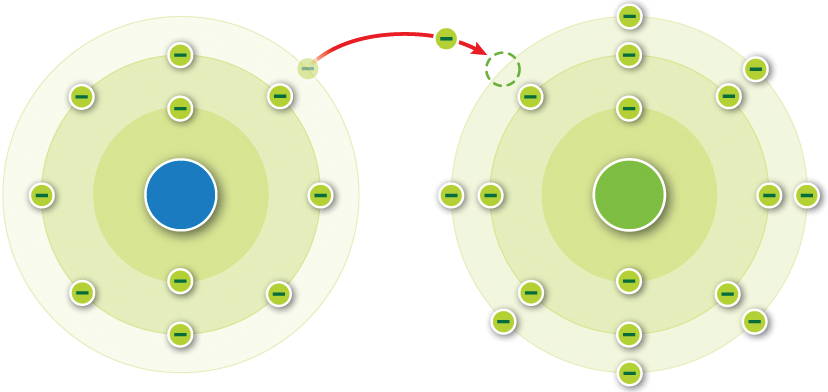 What is the atomic number of the negatively charged ion formed in the diagram?
What is the atomic number of the negatively charged ion formed in the diagram?
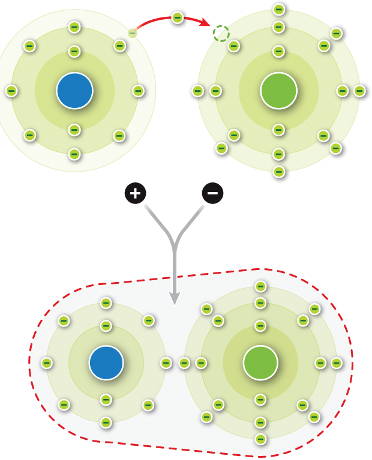 Sodium chloride (NaCl) is composed of alternating sodium and chloride ions held together by ________ bonds.
Sodium chloride (NaCl) is composed of alternating sodium and chloride ions held together by ________ bonds.
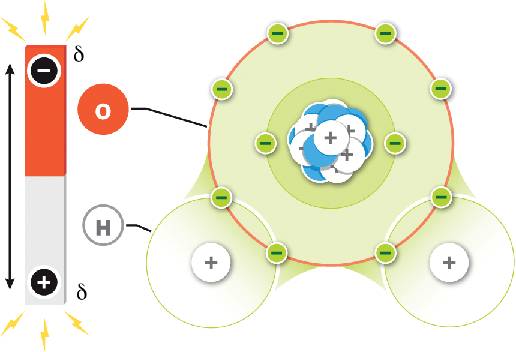 A water molecule has
A water molecule has
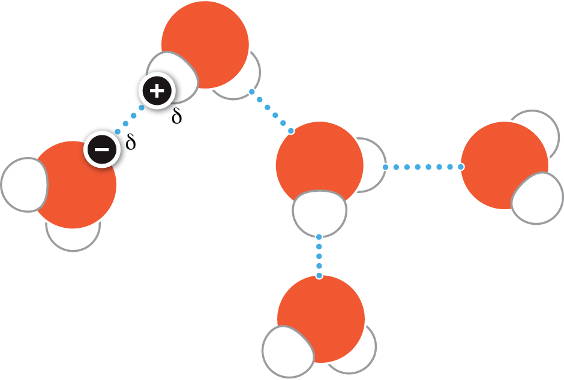 The dotted lines in this diagram are weak ________ bonds due to attraction of partially charged regions between water molecules.
The dotted lines in this diagram are weak ________ bonds due to attraction of partially charged regions between water molecules.
 The property of water that is responsible for surface tension is called
The property of water that is responsible for surface tension is called
| Home | © Copyleft Peter Chen |